Naming molecular compounds may seem complex, but it doesn’t have to be. Understanding the rules and conventions can unlock the mystery behind naming these compounds. The key lies in knowing the elements involved and their positions in the compound. By following a systematic approach, you can confidently tackle naming molecular compounds with ease. This guide will walk you through the essential steps to master the art of naming molecular compounds effectively. Let’s dive in and unravel the secrets of how to name molecular compounds effortlessly.
How to Name Molecular Compounds
The Basics of Naming Molecular Compounds
Naming molecular compounds may sound complicated, but once you understand the simple rules, it becomes easy peasy lemon squeezy! Molecular compounds are made up of two or more non-metal elements bonded together. The names of these compounds follow specific guidelines to help scientists and chemists communicate effectively.
Understanding Compound Formulas
Before we dive into naming molecular compounds, let’s quickly review how to read compound formulas. A compound formula shows the types and numbers of atoms present in the compound. For example, H₂O represents water, with two hydrogen atoms and one oxygen atom.
Types of Atoms in Molecular Compounds
Molecular compounds contain non-metal elements like hydrogen, carbon, nitrogen, oxygen, sulfur, and more. These elements combine in different ratios to form various compounds with unique properties.
Simple Naming Rules for Molecular Compounds
Imagine you have two non-metal elements bonded together. To name this compound, follow these straightforward rules:
1. Naming Binary Molecular Compounds
Binary molecular compounds consist of two elements. The name of the compound always starts with the name of the first element followed by the name of the second element.
For example:
– CO₂ is named carbon dioxide
– N₂O is named dinitrogen monoxide
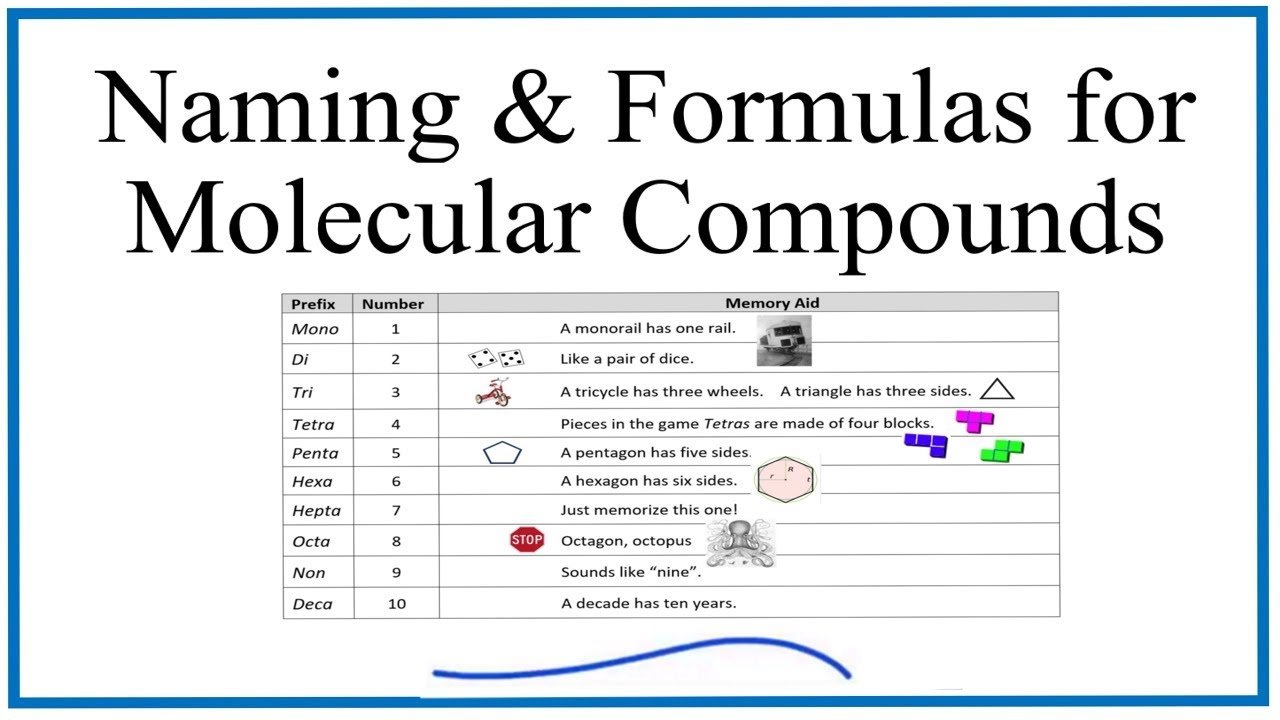
2. Using Prefixes in Naming
Because molecular compounds can have different ratios of elements, we use prefixes to indicate the number of each element present in the compound.
Here are some common prefixes:
– Mono- means one
– Di- means two
– Tri- means three
– Tetra- means four
– Penta- means five
Using these prefixes helps us specify the number of each element in the compound.
Applying Prefixes to Name Compounds
Let’s practice naming some compounds using prefixes:
1. CO
– Since there is one carbon atom and one oxygen atom, we use the prefix “mono” for the first element.
– The compound CO is named carbon monoxide.
2. N₂O₅
– In this compound, there are two nitrogen atoms and five oxygen atoms.
– Using the prefixes, we name this compound dinitrogen pentoxide.
3. P₂O₃
– With two phosphorus atoms and three oxygen atoms, we name this compound diphosphorus trioxide.
Special Cases in Naming Molecular Compounds
Sometimes, you may encounter special cases in naming molecular compounds. Let’s explore some of these unique situations:
1. When to Drop Prefix “Mono”
When the first element only has one atom, we drop the prefix “mono.” For example, CO is carbon monoxide, not monocarbon monoxide.
2. Using Greek Prefixes
For compounds with more than two elements, we use Greek prefixes. For example:
– NaClO₄ is named sodium perchlorate
Greek prefixes help us keep track of the number of each element in complex compounds.
Practice Makes Perfect!
Now that you’ve learned the basic rules for naming molecular compounds, it’s time to practice and master your skills. Remember, practice makes perfect, and soon you’ll be a naming pro!
Naming molecular compounds may seem overwhelming at first, but with a little practice and understanding of the simple rules, you can easily name any compound like a chemistry whiz. Keep exploring the fascinating world of chemistry and enjoy playing with different elements to create unique compounds.
Get ready to impress your friends and teachers with your newfound knowledge of naming molecular compounds!
How To Name Covalent Molecular Compounds – The Easy Way!
Frequently Asked Questions
What are the basic rules for naming molecular compounds?
When naming molecular compounds, the first element is named first using its full elemental name. The second element is named as if it were an anion, with an -ide ending. Prefixes like mono-, di-, tri-, etc., are used to indicate the number of each atom present.
How do you name molecular compounds with two elements?
To name molecular compounds with two elements, you write the name of the first element, then the name of the second element with an -ide ending. Use prefixes to indicate the number of atoms: mono- for one, di- for two, tri- for three, tetra- for four, penta- for five, and so on.
Can you explain how to name molecular compounds with more than two elements?
When naming molecular compounds with more than two elements, identify each element and the number of atoms present. Use the prefixes to indicate the quantity, placing them in front of the element names. Remember to end the name of the second element with an -ide suffix.
Final Thoughts
In conclusion, naming molecular compounds involves following specific rules based on the elements present and their respective charges. Understanding the prefixes for indicating the number of atoms is crucial. Notably, using the correct suffixes for indicating the type of bond between atoms is essential. Remembering the importance of proper naming conventions is key in successfully naming molecular compounds. Mastering how to name molecular compounds will ultimately enhance your understanding of chemistry principles.